Abstract
Background: Proteinuria is observed in >80% of patients with multiple myeloma (MM) and most often consists of free light chains (FLC) and albumin. The standard method of following patients with light-chain MM includes a 24-hour urine collection to determine the degree of monoclonal proteinuria as response criteria are in part based on this parameter. This is often cumbersome, especially in the elderly population and frequently raises concerns for accuracy. An untimed urine collection to provide a spot protein-to-creatinine (SPOT) ratio has been proposed as a reliable and quicker alternative, but its correlation with 24-hour urine protein collection in MM patients is not well established. Our group previously demonstrated a good correlation between the two methods in a small study of 15 patients. We now present the updated results with urine samples from 40 patients to evaluate the correlation between SPOT ratio and 24-hour collection in patients with MM.
Methods: With the approval of the Institutional Review Boards, patients with MM were enrolled in the study after obtaining informed consent. The patients were provided with detailed instructions and supplies to collect a 24-hour urine collection and up to three additional urine samples at different time points during the day. For all the samples submitted, urine protein was measured by benzethonium chloride turbidimetry and creatinine by an IDMS-standardized enzymatic method on a Siemens ADVIA 1800 or the pyrocatechol violet method on the Ortho Vitros 5.1. A linear regression method using R version 3.2.2 was used to correlate values obtained by the two methods. A Bland-Altman plot was used to analyze the limits of agreement between the two methods of measuring proteinuria.
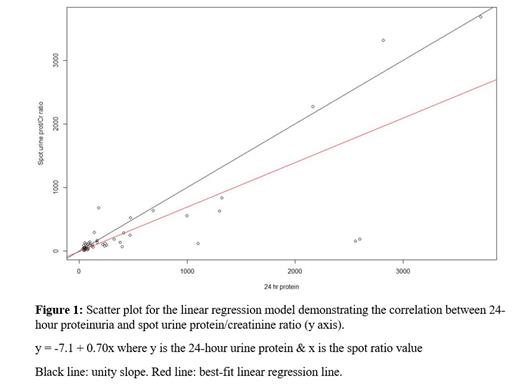
Results: Sixty-four urine samples were obtained for SPOT ratio analysis from 40 patients between 2015-2020. Only complete 24-hour urine samples containing ≥ 0.7 g creatinine were included. The median age of participants was 68.5 [interquartile range (IQR) 59-73] years. Twenty-three (57.5%) of the patients were female. Thirteen patients had only light chain disease, while the remaining 27 patients had either IgG/IgA paired ƙ/ƛ myeloma. Thirty (75%) patients were on therapy at the time of sample collection. A linear regression model of the 24-hour urine protein versus the SPOT ratio was performed. The average 24-hour protein was 457.2 +/- 805.9 mg. The average SPOT ratio was 327.5 +/- 724.0 mg prot/gram Cr. The linear regression line was y = -7.1 + 0.7x where y is the 24-hour urine protein & x is the spot ratio value. The coefficient of the linear regression model was significant (p<0.001). The adjusted R2 value was 0.63 for the model.
Conclusion: In patients with MM, there is a strong correlation between 24-hour urine protein & SPOT ratio, particularly for proteinuria under 1,000 mg. Given its accuracy and lack of inconvenience for the patients, SPOT ratio may be a suitable alternative to 24-hour urine protein collection. In patients with a high SPOT ratio, a 24-hour urine testing should be performed to confirm the results. Before 24-hour urine collection in myeloma can be completely abandoned, studies in larger patient cohorts are needed to differentiate between the monoclonal protein component and other proteins in urine.
Jamshed: Takeda: Honoraria. Hillengass: GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Beijing Medical Award Foundation: Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees; Axxess Network: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Beijing Life Oasis Public Service Center: Speakers Bureau; Curio Science: Speakers Bureau; Adaptive: Membership on an entity's Board of Directors or advisory committees; Skyline: Membership on an entity's Board of Directors or advisory committees; Oncotracker: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal